Charcot-Marie Tooth disease:
A 100% French RNA-based therapeutic innovation
Charcot-Marie Tooth disease is the most common hereditary neurological disease in the world. It affects the peripheral nerves and causes progressive paralysis of the legs and hands. No treatment is currently available to fight this disease, which is due to the overexpression of a specific protein, peripheral myelin protein of 22 kDa (PMP22). When PMP22 is made twice as much as normal, it causes type 1A of genetic Charcot-Marie Tooth disease to develop. This overproduction leads to gradual paralysis of the legs and hands. Scientists from the CNRS, INSERM, the AP-HP and the Paris-Saclay and Paris universities have developed a therapy based on degrading the coding RNA for this protein in mice. Their work is patented and was published on 9 March 2021 in Communications Biology (1). These scientists have developed a patented therapy (WO/2020/064749, PCT/EP2019/075736) based on reducing RNA coding for the PMP22 protein. To achieve this they used small interfering (si) RNA molecules capable of interfering with mRNA that encodes PMP22, and of degrading or reducing its translation into protein. The difficulty in developing this therapy has been to stabilise these small RNAs which degrade very rapidly in biological environments.
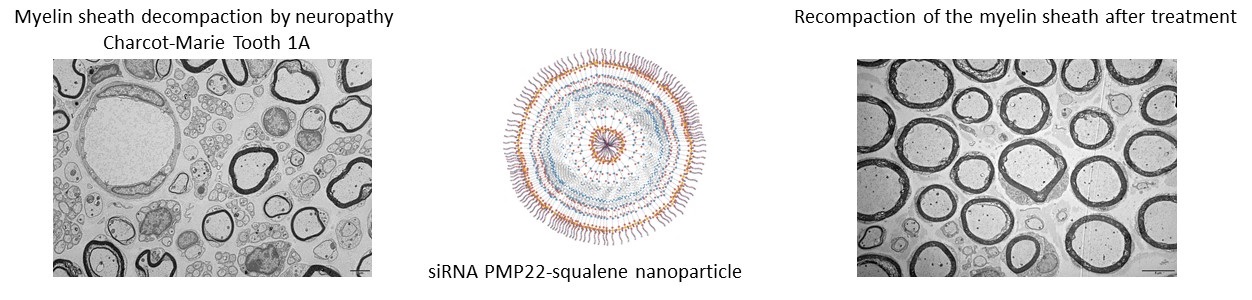
Myelin sheath decompaction by neuropathy Charcot-Marie Tooth 1A siRNA PMP22-squalene nanoparticle Recompaction of the myelin sheath after treatment. © Liliane Massade, Diseases and hormones of the nervous system (INSERM/Université Paris-Saclay)
Researchers have coupled their siRNA with squalene, which is typically used in cosmetology and pharmacology. Biocompatible, biodegradable and forming nanoparticles in water, squalene protects siRNA from degradation. It also controls the size of the particles formed and the amount of siRNA injected. These scientists then showed, in mice models for this disease, that injecting these interfering RNAs completely and rapidly restored of mouse locomotor activity and strength. The siRNAs penetrate the peripheral nerves, strengthen the myelin sheath around those nerves, and normalize the nerve signal velocity. The effect of treatment lasts for three weeks for severe forms and more than ten weeks for milder forms of the disease.This therapeutic strategy for hereditary peripheral neuropathies, developed entirely in France, is proof of concept for a new precision medicine based on siRNA normalization of the expression of an overexpressed gene. It will now be developed in humans with pre-clinical and clinical studies.
(1) Squalenoyl siRNA PMP22 nanoparticles are effective in treating mouse models of Charcot-Marie-Tooth disease type 1 A. Boutary, S., Caillaud, M., El Madani, M. et al. Commun Biol 4, 317 (2021). https://doi.org/10.1038/s42003-021-01839-2. Liliane Massade+33 1 49 59 18 30. liliane.massade@inserm.fr
Anne-Lise Berthier (09/03/2021)




COMMENTS ARE OFF THIS POST