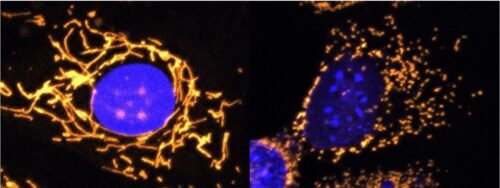
A new gene therapy could pave the way to the treatment of mitochondrial dysfunctions Scientists from Trinity College Dublin have developed a new gene therapy approach that offers promise for one day treating dominant optic atrophy (DOA). This eye disease is characterized by degeneration of the optic nerves and primarily…..
AlgoTherapeutix raises €12M to fight against chemotherapy-induced peripheral neuropathy France-based biotechnology company AlgoTherapeutix has raised €12M in a Series A round led by Bpifrance through its InnoBio 2 fund with co-investor Omnes. The proceeds will be used to fund the clinical development of its lead candidate ATX01 up to clinical…..
Record financing for the regenerative medicine sector through Q3 2020 The regenerative medicine sector attracted $15.9 billion in financing through just the first three quarters of the year, shattering the previous record of $13.5 billion Global financing for the regenerative medicine and advanced therapy sector set an annual record of…..
Ultragenyx will build a new gene therapy manufacturing facility in Bedford Ultragenyx Pharmaceutical, a US biopharmaceutical company focused on the identification, acquisition, development, and commercialization of novel products for the treatment of rare and ultra-rare genetic diseases, plans to build a new large-scale gene therapy manufacturing facility in Bedford, Massachusetts.…..
Le site Sanofi Genzyme de Lyon Gerland devient la première plateforme mondiale Sanofi pour la thérapie génique Quelques jours après le rachat de la biotech néerlandaise Kiadis Pharma et de sa plateforme d'immunothérapies à base de cellules NK (Natural Killer) pour 308 millions d'euros, Sanofi annonce maintenant le lancement d'un…..
Minaris Regenerative Medicine will produce Mustang Bio's MB107 lentiviral gene therapy for the treatment of SCID Mustang Bio, a US clinical-stage biopharmaceutical company focused on cell and gene therapies for the treatment of hematologic cancers, solid tumors and rare genetic diseases, and Minaris Regenerative Medicine, a leading global contract development…..
New CEO for Polyplus-transfection Polyplus-transfection, a leading biotechnology company that supports the gene and cell therapy market by supplying innovative transfection solutions, named Mario Philips as Chief Executive Officer. He succeeds Karsten Wilking, who will transition into a strategic advisor role. Prior to this appointment, Mario Philips served as CEO of…..
EIB backs Atriva Therapeutics with €24 million for the development of a potential COVID-19 treatment
EIB backs Atriva Therapeutics with €24 million for the development of a potential COVID-19 treatment The European Investment Bank (EIB) and Atriva Therapeutics, a german biopharmaceutical company pioneering the development of host-targeting antiviral therapies, concluded a €24 million financing agreement to facilitate the company’s development and clinical testing of…..
Positive CHMP Opinion for Libmeldy™ for the Treatment of Early-Onset Metachromatic Leukodystrophy (MLD) The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has adopted a positive opinion recommending full, or standard, marketing authorization for Libmeldy® (cryopreserved autologous CD34+ cells encoding the arylsulfatase-A, or ARSA,…..
NIH begins large clinical trial to test immune modulators for treatment of COVID-19 The National Institutes of Health (NIH) has launched an adaptive Phase 3 clinical trial to evaluate the safety and efficacy of three immune modulator drugs in hospitalized adults with COVID-19. Some COVID-19 patients experience an immune response…..
- Page Précédente
- 1
...
- 10
- 11
- 12
- 13
- 14
...
- 39
- Page Suivante