A new gene therapy could pave the way to
the treatment of mitochondrial dysfunctions
Scientists from Trinity College Dublin have developed a new gene therapy approach that offers promise for one day treating dominant optic atrophy (DOA). This eye disease is characterized by degeneration of the optic nerves and primarily targets the retinal ganglion cells, leading to a progressive loss of vision. DOA typically starts to cause symptoms in patients in their early adult years. These include moderate vision loss and some colour vision defects, but severity varies, symptoms can worsen over time and some people may become blind. There is currently no way to prevent or cure DOA. Mutations in the OPA1 gene that provides instructions for making a mitochondrial dynamin like GTPase cause severe mitonchondrial dysfunction and are responsible for the onset and progression of the disorder. The OPA1 protein is involved in oxidative phosphorylation, from which cells derive much of their energy. Additionally, this protein plays a role in the maintenance of the DNA within mitochondria and in apoptosis.
An AAV-based gene therapy allowing to boost mitochondria’s functions
Scientists, led by Dr Daniel Maloney and Professor Jane Farrar from Trinity’s School of Genetics and Microbiology, have developed an AAV-based gene therapy that delivers two codon optimized OPA1 isoforms, 1 and 7. They have explored its potential in a range of in vitro and in vivo models, including DOA patient derived fibroblasts and mice treated which a chemical targeting the mitochondria. Their results show that AAV-mediated intravitreal delivery successfully protected the visual function of these mice. DOA patient derived fibroblasts treated with the same AAVs showed a significant improvement in mitochondrial bioenergetics. Dr Maloney, Research Fellow, said: “We used a clever lab technique that allows scientists to provide a specific gene to cells that need it using specially engineered non-harmful viruses. This allowed us to directly alter the functioning of the mitochondria in the cells we treated, boosting their ability to produce energy which in turn helps protects them from cell damage.
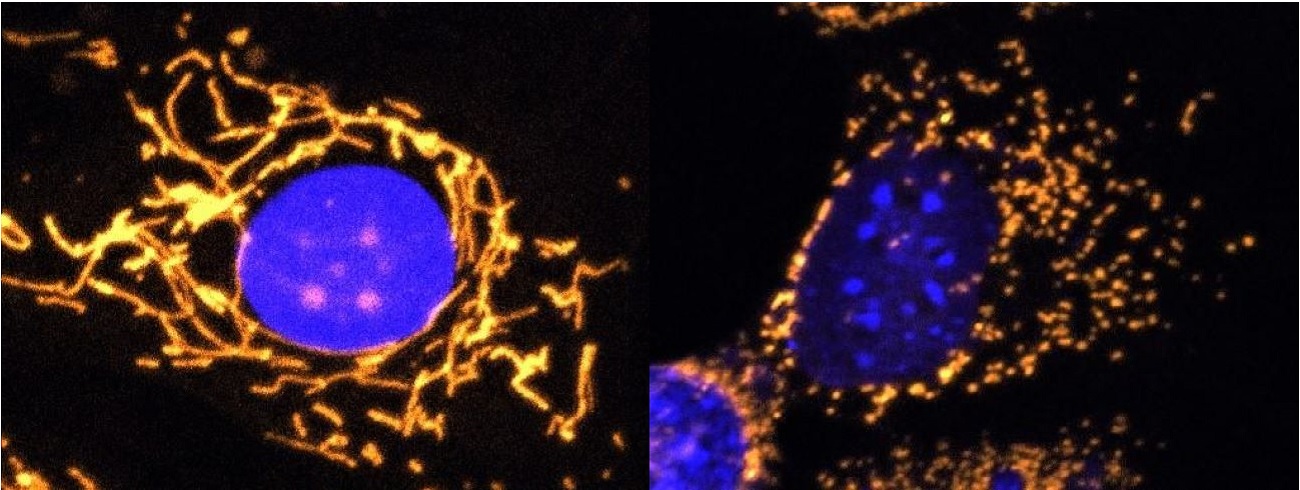
Healthy cell and cell lacking OPA1 protein
Left: Fluorescent microscope image with mitochondria highlighted in gold. This healthy cell shows a highly elaborate and well-connected network of mitochondria.
Right: Fluorescent microscope image with mitochondria highlighted in gold. This cell completely lacks OPA1 protein and shows fragmented mitochondria.
©Professor Jane Farrar and Dr Daniel Maloney, Trinity College Dublin
“Excitingly, our results demonstrate that this OPA1-based gene therapy can potentially provide benefit for diseases like DOA, which are due to OPA1 mutations, and also possibly for a wider array of diseases involving mitochondrial dysfunction.” Importantly, mitochondrial dysfunction causes problems in a suite of other neurological disorders such as Alzheimer’s and Parkinson’s disease. So these results could have implications for a much wider suite of neurological disorders associated with ageing. Professor Farrar, Research Professor, added: “OPA1 mutations are involved in DOA and so this OPA1-based therapeutic approach is relevant to DOA. However mitochondrial dysfunction is implicated in many neurological disorders that collectively affect millions of people worldwide. We think there is great potential for this type of therapeutic strategy targeting mitochondrial dysfunction to provide benefit and thereby make a major societal impact. Having worked together with patients over many years who live with visual and neurological disorders it would be a privilege to play a role in a treatment that may one day help many.“
The study, which involved a collaboration with clinical teams in the Royal Victoria Eye and Ear Hospital and the Mater Hospital, has been published in Frontiers in Neuroscience.
Anne-Lise Berthier (27/11/2020)



COMMENTS ARE OFF THIS POST