Date: 2017-03-27
Type of
information: Results
phase: 2
Announcement: results
Company: Diamyd Medical (Sweden)
Product: combination of Diamyd® with relatively high doses of vitamin D and ibuprofen
Action
mechanism:
- protein. Diamyd® is an antigen-based diabetes therapy under development to prevent, delay, or stop the autoimmune attack on beta cells in type 1 diabetes and other forms of autoimmune diabetes, thereby preserving the body's capacity to regulate blood sugar. The active substance in the Diamyd® diabetes vaccine is glutamic acid decarboxylase isoform 65kDa (GAD). GAD is one of the most important targets when the immune system attacks the beta cells in autoimmune diabetes. Accordingly, GAD is an autoantigen. Treatment using Diamyd® is intended to stop the autoimmune attack against the beta cells by inducing tolerance to GAD.
Disease: type 1 diabetes
Therapeutic
area: Autoimmune diseases - Metabolical diseases
Country: Sweden
Trial
details:
- DIABGAD-1 is a double-blind, randomized and placebo-controlled Phase II study including a total of 64 participants between 10 and 18 years old, newly diagnosed with type 1 diabetes. Patients have been randomized to four treatment groups, for which C-peptide data is available for 50 patients at 15 months. The first group received one injection of Diamyd® 20µg on two occasions four weeks apart combined with ibuprofen over the course of 90 days, in addition to vitamin D for a period of 15 months; the second group received one injection of Diamyd® 20µg on two occasions four weeks apart combined with vitamin D for a period of 15 months; the third group received a double dose of Diamyd® four weeks apart and vitamin D for 15 months and; the fourth group received placebo only. Enrollment in the study, which is double-blind, randomized and placebo-controlled, commenced in February 2013. All participants have now been monitored for 15 months of the 30-month total for the study.
- The trial has been conducted at nine pediatric diabetes clinics in Sweden with Professor Johnny Ludvigsson, Linköping University, as the principal investigator and sponsor.
Latest
news:
- • On March 27, 2017, Diamyd Medical announced that 30 month results from DIABGAD-1 support effect after partial remission phase. Results after 30 months of this investigator initiated pilot trial show that treatment with the diabetes vaccine Diamyd® in combination with vitamin D and ibuprofen has a good safety profile and no serious related side effects. With the entire trial period taken into account, no significant difference in the ability to produce insulin was seen between the patient groups receiving active and placebo treatment. However, after the initial period, (partial remission phase or honeymoon period), a significant positive clinical effect was seen in the group receiving active treatment compared with placebo, and the effect was largest in the group reciving a double dose of Diamyd® plus vitamin D.
- “Over the course of the entire trial period the diabetes vaccine, which in this trial was given subcutaneously in combination with vitamin D and ibuprofen, did not show an improvement in the effects of earlier studies with Diamyd®. The positive effect seen between 6 and 15 months has endured and has become significant at 30 months when looking at the difference in decline of the endogenous insulin-producing ability compared to placebo. This is really positive and something that we will further analyze in detail,” says Professor Johnny Ludvigsson at Linköping University, principal investigator of the trial.
- Viewed over the entire 30 month trial period, no significant difference was seen between the placebo and the active groups, which all show a decrease in the ability to produce insulin (measured as C-peptide Area Under the Curve (AUC) in nmol/L). However, between 6 and 30 months (i.e. the partial remission phase, or honeymoon period), a significant difference in the decline of C-peptide AUC was seen between the groups receiving active and placebo treatment. Looking at the individual groups, those patients receiving a double dose of Diamyd® also declined significantly in the marker of blood sugar over time, HbA1c, compared to the placebo group between 6 and 30 months.“These results provide further support for the positive effects and safety of Diamyd® in recent onset type 1-diabetic patients. It seems, however, that the observed effect in sustaining the endogenous insulin producing ability in newly diagnosed type 1-diabetes shown in DIABGAD-1 is limited, but can be significantly improved in the ongoing investigator-initiated DIAGNODE-1 trial, where the diabetes vaccine is administered directly into the lymph node, and preliminary results are very promising. In the light of this, our investment into our own clinical trial with intralymphatic injections of Diamyd® are spot on”, says Ulf Hannelius, CEO of Diamyd Medical.
- • On September 7, 2016, Diamyd Medical provided update on clinical studies with Diamyd® for the treatment and prevention of type 1 diabetes. The placebo-controlled study DIABGAD is now fully enrolled and results are expected in the first quarter of 2017.• On December 17, 2015, Diamyd Medical announced from a clinical investigator-initiated pilot study, DIABGAD-1, that the diabetes vaccine Diamyd® in combination with vitamin D and ibuprofen after 15 months has a good safety profile with no reported serious side effects related to the treatment. The data shows that after the initial phase (referred to partial remission or the honeymoon phase), the group receiving placebo (non-active substance) lost their ability to produce insulin at a rate that was 2-3 times faster during the last 9 months of the 15-month period, compared with the groups receiving active treatment with Diamyd®. However, viewed over the entire 15-month period no difference between the groups is observed, but if the more rapid decrease continues in the placebo group until the end of the study at 30 months, a trend deviation in insulin production may be observable throughout the full measurement period, that is, including the remission period.
The DIABGAD-1 study is conducted at nine pediatric diabetes clinics in Sweden, with Professor Johnny Ludvigsson, Linköping University, as the principal investigator and sponsor. The purpose of the pilot study is to test the combination therapies’ safety and how they impact the body’s own ability to produce insulin in children and adolescents newly diagnosed with type 1 diabetes. The participants will be monitored for 30 months. The preliminary 15-month results indicate that the safety of the combination therapy is good and no serious adverse events related to the treatment have been reported.
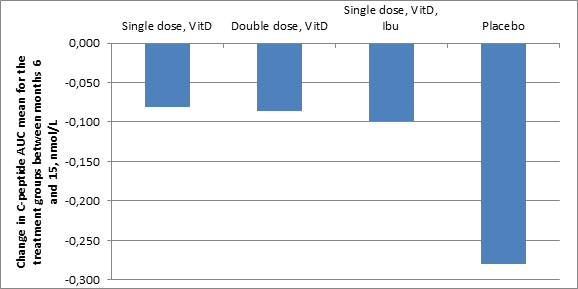
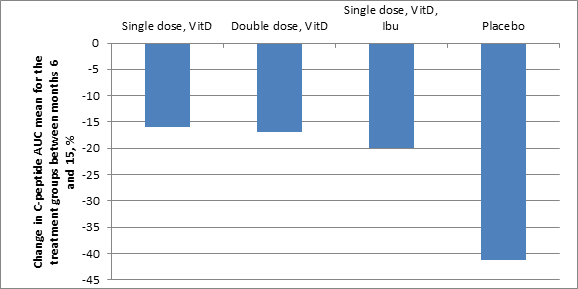
Following the start of insulin treatment, many type 1 diabetes patients enter partial remission which, depending on age, can extend over a period of a few months up to a couple of years (Pecheur et al., J Diabetes Research, 2014:851378). For this reason, the effects of the various combination therapies is presented below as the difference in the insulin-producing capacity between the date for the 6-month visit after inclusion in the study, which is close to the mean value for the length of the stable phase (partial remission), and the visit 15 months after inclusion in the study. Over the course of these 9 months, the ability to produce insulin (measured as C-peptide Area Under the Curve (AUC) in nmol/L) decreases in the placebo group at a rate that is 2-3 times faster than the groups treated with Diamyd®. If this more rapid decrease continues among the placebo group until the end of the study at 30 months, a trend deviation in insulin production may be observable throughout the complete measurement period, that is, including the remission period.
From the date of inclusion up to 15 months, meaning including the estimated remission period, no difference in insulin production, measured as C-peptide AUC, is observable between the groups. However, measured from month 6 to month 15, the capacity to produce insulin declined in the placebo group (n=10) by 0.28 nmol/L (41%), while the group that received Diamyd®, vitamin D and ibuprofen (n=11) declined by 0.10 nmol/L (20%); the group receiving Diamyd® and vitamin D (n=15) declined by 0.08 nmol/L (16%), and the group that received a double dose of Diamyd® and vitamin D (n=14) declined by 0.09 nmol/L (17%).
 In a previous Phase III study (n=334), treatment with the diabetes vaccine Diamyd® alone was shown to yield a positive trend (16% effect, p=0.1) in terms of the patients’ own ability to produce insulin. To achieve a better effect, the diabetes vaccine is therefore being tested as part of various combination therapies in several different pilot studies. The efficacy and safety of initially positive combinations should thereafter be validated in larger studies in order to be approved as a treatment.
In a previous Phase III study (n=334), treatment with the diabetes vaccine Diamyd® alone was shown to yield a positive trend (16% effect, p=0.1) in terms of the patients’ own ability to produce insulin. To achieve a better effect, the diabetes vaccine is therefore being tested as part of various combination therapies in several different pilot studies. The efficacy and safety of initially positive combinations should thereafter be validated in larger studies in order to be approved as a treatment.- • On June 9, 2014, Diamyd has announced that all participants have been included in a Phase II clinical study, DIABGAD-1, in which diabetes vaccine Diamyd®, in a unique combination with other drugs, is tested in children and adolescents recently diagnosed with type 1 diabetes. The first results from the researcher initiated study will thereby be available in the beginning of 2015. DIABGAD-1 is the first study of its kind, it combines the diabetes vaccine Diamyd® with vitamin D and the anti-inflammatory drug ibuprofen. The Phase II study also evaluates the effect of a double dose of Diamyd® and the protein GAD, which is the active substance in Diamyd®. The purpose of the treatment is to preserve the body's own insulin producing capacity and ability to control the blood sugar level in children and adolescents newly diagnosed with type 1 diabetes. Since the final participant now has been included, the first analysis will be initiated at the end of 2014 and the results can be presented in the beginning of 2015.
“We strongly believe in attacking the disease process underlying type 1 diabetes simultaneously from several angels through the combination of our diabetes vaccine Diamyd® with other therapeutics, and this is one of the first such studies in the world,” says Peter Zerhouni, President and CEO of Diamyd Medical. “I am very happy and grateful that so many children and adolescents with their families have chosen to participate in the study and also for the strong commitment from the clinical researchers and other staff.” The study is conducted at nine pediatric diabetes clinics in Sweden. It is researcher initiated and led by Professor Johnny Ludvigsson at Linköping University.
- • On January 30, 2013, Diamyd Medical has announced that a new clinical study with the diabetes vaccine Diamyd® is planned to start in February 2013. In the study Diamyd® will be tested in a unique combination with other drugs, aiming to potentiate the effect of the diabetes vaccine. The Company has entered into an agreement with Linköping University to conduct the researcher-initiated study. The study has been approved by the Swedish Medical Products Agency. The study is called DIABGAD-1 and combines the diabetes vaccine Diamyd® with relatively high doses of vitamin D and the anti-inflammatory drug ibuprofen. The purpose of the treatment is to preserve the body's own ability to control the blood sugar level in children and adolescents newly diagnosed with type 1 diabetes.
- The study will also evaluate the effect of a double dose of Diamyd® and the protein GAD, which is the active substance in Diamyd®. The study will include 60 children and adolescents in Sweden and it will be conducted at pediatric diabetes clinics in Malmö, Lund, Halmstad, Kalmar, Jönköping, Uddevalla, Örebro, Linköping and Stockholm.
- The study is funded by research grants, while Diamyd Medical is responsible for providing study drug and certain other costs, and can utilize the study results. Since 2009 the diabetes vaccine Diamyd® is being evaluated in a Swedish researcher-initiated Phase II study, DiAPREV-IT. That study includes a total of 50 children aged four and older who, through analysis of diabetes markers in the blood, are demonstrated to be at high risk of developing type 1 diabetes, but have not yet presented with disease. The purpose of the study is to evaluate whether preventive treatment with Diamyd®, compared to placebo, can delay or halt the progression of the disease so that the children do not develop clinical symptoms of type 1 diabetes. The first results are expected to be compiled in 2015.
Is
general: Yes

 In a previous Phase III study (n=334), treatment with the diabetes vaccine Diamyd® alone was shown to yield a positive trend (16% effect, p=0.1) in terms of the patients’ own ability to produce insulin. To achieve a better effect, the diabetes vaccine is therefore being tested as part of various combination therapies in several different pilot studies. The efficacy and safety of initially positive combinations should thereafter be validated in larger studies in order to be approved as a treatment.
In a previous Phase III study (n=334), treatment with the diabetes vaccine Diamyd® alone was shown to yield a positive trend (16% effect, p=0.1) in terms of the patients’ own ability to produce insulin. To achieve a better effect, the diabetes vaccine is therefore being tested as part of various combination therapies in several different pilot studies. The efficacy and safety of initially positive combinations should thereafter be validated in larger studies in order to be approved as a treatment.